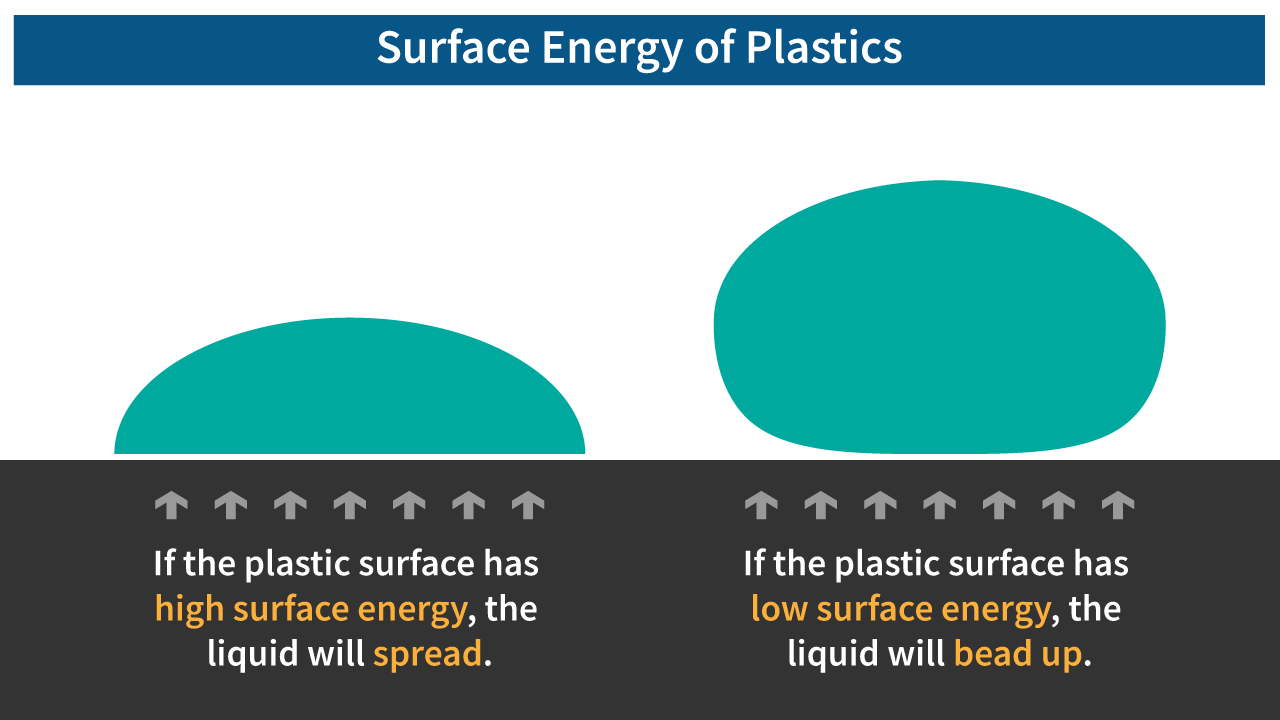
Surface energy in plastics quantifies the disruption of intermolecular bonds in the material. A higher value indicates a stronger affinity between molecules. This value helps us determine how readily these molecules bond to other substances.
When working with any polymer, if the material surface energy is relatively low, then any coating will not flow well and fisheyes, pinholes, gaps, or air bubbles can form. If the material surface energy is too high, then the paint, ink, or coating may bleed or be difficult to control. Therefore, the surface tension of the liquid and the surface energy of the material must be matched for the application.
The Dynamics of Wetting are Described Below
Spreading = A - ( B + C )
Where:
- A = Surface energy of solid (given below)
- B = Surface tension of liquid
- C = Surface energy of solid-liquid interface
If Spreading is:
- Negative - Then, liquid will bead up.
- Zero to Positive - Then, liquid will spread.
Surface Energy of Common Polymers
The table below shows the relative surface energy (in dynes/cm) of some common polymer materials.
| Polymer Abbr. | Polymer Name | Surface Energy (dynes/cm) | Contact Angles (degrees) |
|---|---|---|---|
| PES | Polyethersulfone | 46 | 90 |
| Styrene butadiene rubber | 48 | ||
| PPO | Polyphenylene oxide | 47 | 75 |
| Nylon 6/6 (polyhexamethylene adipamide) | 46 | ||
| PC | Polycarbonate | 46 | 75 |
| Nylon-6 (polycaprolactam) | 38 | ||
| PET | Polyethylene terephthalate | 42 | 76 |
| PMMA | Polymethylmethacrylate | 41 | 82 |
| SAN | Styrene acrylonitrile | 40 | 74 |
| Polyimide | 40 | 83 | |
| PVC r | Polyvinyl chloride, rigid | 39 | 90 |
| Polyester | 41 | 70 | |
| Acetal | 36 | 85 | |
| ABS | Acrylonitrile butadiene styrene | 35 | 82 |
| PPS | Polyphenylene sulfide | 38 | 87 |
| PVA | Polyvinyl alcohol | 37 | 10 |
| Polyacrylate (acrylic film) | 35 | ||
| PVC p | Polyvinyl chloride, plasticized | 35 | 89 |
| PS | Polystyrene | 34 | 72 |
| Nylon-12 | 36 | ||
| Surlyn ionomer | 33 | 80 | |
| PBT | Polybutylene teraphthalate | 32 | 88 |
| CTFE | Polychlorotrifluoroethylene | 31 | |
| PP | Polypropylene | 30 | 88 |
| PU | Polyurethane | 38 | 85 |
| PE | Polyethylene | 30 | 88 |
| PVF | Polyvinyl fluoride | 28 | |
| PVDF | Polyvinylidene fluoride | 25 | 80 |
| Natural rubber | 24 | ||
| PDMS | Polydimethyl siloxane (silicone elastomer) | 23 | 98 |
| FEP | Fluorinated ethylene propylene | 20 | 98 |
| PTFE | Polytetrafluoroethylene | 19 | 120 |
Methods for Improving Surface Energy
To improve the surface energy of plastics and make them more amenable to adhesion, printing, or other applications, methods such as corona, flame, or plasma surface treatment are often used. These processes modify the surface chemistry and increase its energy, making the plastic more receptive to coatings, inks, or adhesives.
Watch our video for more on this topic: